Technology Offers Lab Animals a Way Out
Quality Growth Boutique
ESG concerns over climate change, water security and plastic pollution have overtaken the airwaves of late – but you’d be mistaken to dismiss animal testing as a bygone controversy. Despite bans and restrictions, an estimated1 10 million animals are still put through tests involving severe pain every year. All for the benefit of mankind and in the name of beauty and medical research – some is warranted till better methods are proven, but much isn’t. It’s a barbarous method and the time will come when it’s no longer viable, replaced by the rapid advances in technology and science. Unfortunately, that time might not be soon. But the bogeyman has changed since the 1990s and regulators have become part of the problem.
The world’s first ban on animal testing on cosmetic products was passed in the European Union in 1993 and the region has been considered the gold standard for such regulations. Animal testing for cosmetics has also been banned in India, Israel, Norway, Switzerland and beyond. In the United States, while animal testing is not required for cosmetics, it’s not banned either. Other markets have lagged the leaders, and if animal testing is required for just one market a product is sold in – progress is defeated.
An EU-wide moratorium let alone a global one on the use of animals in science has not so far been possible. In fact, because of the Covid-19 pandemic, the medical community writ large has appealed to regulators globally to reconsider the importance of animal testing as essential for the advancement of human and veterinary health and to save lives.
Meanwhile, and on the other side of that debate, you’ll find the actor and avid animal rights activist Ricky Gervais who recently joined Humane Society International in bringing fresh visibility to this hot-button topic. The animated campaign featuring Ralph the test rabbit is designed to further awareness, and tug on heartstrings. The controversy rages on. Click on the photo to see the animation.

There is, however, some good news. Advances in alternative testing methods using AI and devices have progressed. New safety testing standards called New Approach Methodologies (NAMS) are non-animal technologies that either use a human cell in vitro or “lab-on-a-chip” technologies where a machine conducts the same medical analysis it would on a living being. These new tools have been boosted recently as new regulations supporting the use case in the United States and elsewhere have taken hold.
These advances are shining a light into the future as they will provide an added incentive to move away from animal testing with more accurate and quicker results. Because beyond the ethical questions there is the constant problem using animals for testing that they do not necessarily mimic how a human body would react accurately – notably when dealing with toxicology. The outlook for change improves when there’s a business driver.
Regulation and a breakthrough in China
One of the major bottlenecks to reducing animal testing has become slow moving regulation. Regulators are often conservative and sometimes not motivated to take risks at a personal level. One major regulatory bottleneck has been the Chinese requirement to test imported cosmetics on animals. But following extensive talks between government officials, a number of large consumer goods companies and NGOs, the Chinese authorities announced that the sale of most imported “general cosmetics” (such as shampoo, lipstick, and makeup) can go ahead without being tested on animals. This new rule went into effect in May 2021. The rule does not ban testing for all cosmetics. Special use cosmetics such as sunscreen and hair dyes will continue to require testing. But the important point is that successful lobbying helped to drive this big step forward. As a result, a number of cruelty free brands will be able to enter the market.
Still, new regulations and technologies haven’t eliminated animal testing practices across the board, which is why those companies that use such techniques must realize that they risk a highly visible litmus test of corporate values. Companies associated with poor values can suffer brand damage and attract hard hitting NGOs who seek to make examples out of worst practices. Companies that test products on bunnies and other furry creatures are easy targets.
Who’s exposed?
I have used business involvement figures for animal testing from Sustainalytics. The chart below shows 448 companies flagged within the MSCI All Country World Index (MSCI ACWI). Unsurprisingly it’s concentrated with just under half of the companies using such testing coming from the Heath Care sector, and just over nine out of ten companies from the top four sectors. The companies are split between those with pharmaceutical products (60% of the total) and non-pharmaceutical products. The leading countries in this set are the US (23% of total), China (20%) and Japan (15%) due to their sizable (and listed) health care sectors. It’s worth noting that around a fifth of the flagged companies are categorized as “suspected involvement” due to lack of disclosures but because they operate in areas where animal testing is typically in use.
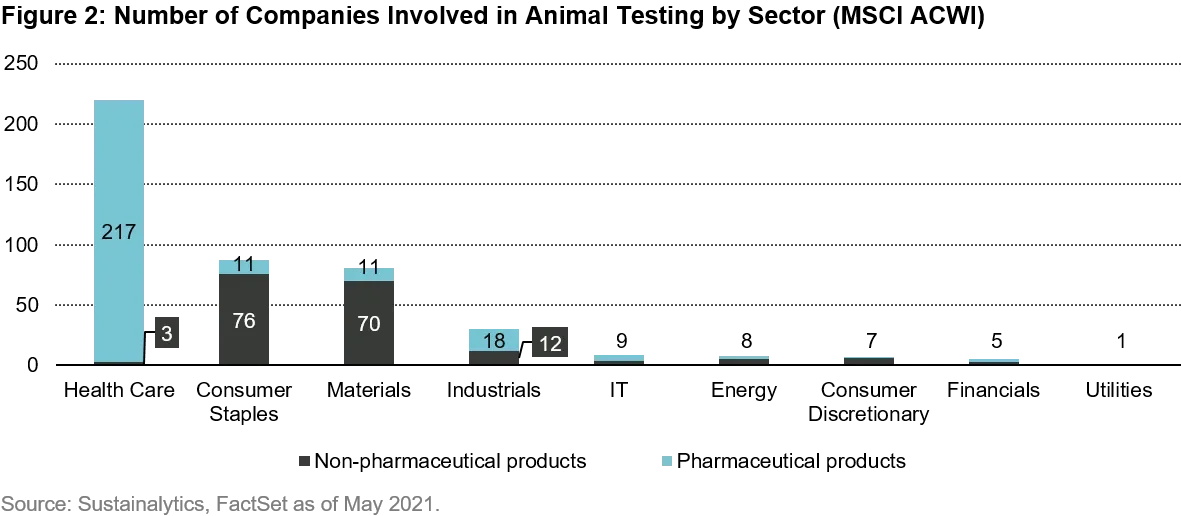
It’s important to note that pharmaceutical products impact human health through compounds for medicine and medical devices. Licenses to sell in a market can often only be obtained following tests for safety, often involving lab animals. Some products will be used by large numbers of people, and the leveraged benefit of effective safety tests can be significant. Due to the public interest, the industry is heavily regulated. But there are many new technologies that in the long term are likely to remove the need for most animal testing.
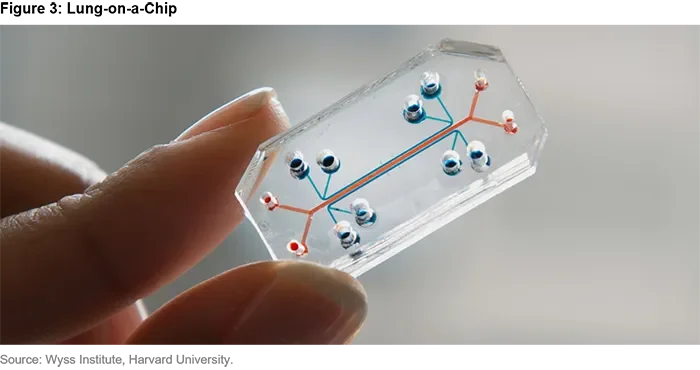
These include devices that replicate human responses such as Organs-on-Chips2 researched by the likes of Harvard University and the European Organ-on-Chip Society, and Tissue Chips3 backed by the U.S. National Center for Advancing Translational Sciences (NCATS). Technological progress has been illustrated by the rapid development of the high efficacy Moderna and Pfizer/BioNTech mRNA Covid-19 vaccinations. These were tested on humans at the same time they were tested on animals (due to urgency).
There are also safety tests for ingredients and chemicals used in products ranging from food and cosmetics, to industrial adhesives, paint, and pesticides. Industrial chemicals can be dangerous and as a result are generally tightly regulated for safety. Some require the use of animal tests. Cosmetics is generally seen as the low hanging fruit for regulatory advances due to lighter regulation in the large EU and US markets, and ingredients often fall under different rules. The challenge for customer facing multi-national companies is while regulators are local, public perception is global. Animal testing for one market is still animal testing.
Management discussions
Following are some discussions I’ve had with companies about animal testing. For a sense of how managements explain the positions they are in, the first observation is that animal testing remains a sensitive topic and there are few required disclosures. The second is that most companies point to the broad 3R goals originally laid out by the EU4 on the protection of animals used for scientific purposes:
- Replace: the use of animals with other methods
- Reduce: the number of animals used
- Refine: procedures to minimize the impact to animals.
Boston Scientific, the US health care equipment and supplies company, tests on animals for research and development purposes as well as for regulatory submissions. They use non-animal models when possible as an alternative, including: in vitro and ex vitro (both testing done outside an animal), human cadaver, synthetic and computer based simulations. Coca Cola’s management pointed to animal testing, undertaken by third parties, required to verify the safety of ingredients and additives in some markets. Again, using alternatives to animal testing whenever suitable to demonstrate safety.
Unilever, the multinational consumer goods company producing cosmetics, food and drinks, and home care products, acknowledged they and parts of their supply chain are required to animal test for product safety in certain markets including Russia and China. The company is a vocal supporter of ending animal testing, and back the Humane Society International’s campaign for regulatory change on animal testing. They have engaged with politicians and policy makers including pushing back against calls for new animal testing by the European Chemicals Agency.
For French luxury clothing drinks and cosmetics company LVMH, animal testing has primarily impacted its China cosmetics business with the Guerlain, Givenchy and DIOR brands. The company runs its own in vitro toxicology department that runs tests on raw materials and ingredients in-house using alternatives to animals testing for cosmetics. LVMH has worked with the Chinese authorities to change the rules on imported products as well.
Pest control company Rentokil Initial is less typical given its primary business is to control insects and pests around commercial and residential buildings. The pest control business reduces the chances of spreading disease in human populations and protects property from damage. Rentokil uses animal testing to register rodenticide products. Most countries the company operates in require studies to show the effectiveness of rodenticide, insecticide, repellent or attractant - both in controlled lab studies and in realistic field settings. The company says it is looking to introduce more humane tools where possible – and pointed to the improved standards for rodent trap design. Of note, the company is rated AA by MSCI ESG at the time of writing.
Summary
The practice of testing on animals is undesirable. Decades of guerilla marketing by hard hitting animal rights NGOs and others has left a lasting impression with consumers and on business values. Now, a broad coalition of companies, NGOs and forward-thinking lawmakers are working together to find alternatives. Luckily, the technologists are also on the case. While realistically a longer-term challenge, breakthroughs in AI and simulation science is our best hope to put this business practice behind us once and for all.
1.
https://www.rspca.org.uk/adviceandwelfare/laboratory
2.
https://wyss.harvard.edu/media-post/lung-on-a-chip/
3.
https://ncats.nih.gov/files/Tissue-Chip-factsheet.pdf
4.
https://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm
Disclosure
The discussion of any investments in this paper is for illustrative purposes only and there is no assurance that the adviser will make any investments with the same or similar characteristics as any investments presented. The investments identified and described do not represent all of the investments purchased or sold for client accounts. The representative investments discussed were selected based on topics related to our ESG research. The reader should not assume that an investment identified was or will be profitable. There is no assurance that any investments identified will remain in client accounts at the time you receive this document.
Certain information ©2021 MSCI ESG Research LLC. This report contains “Information” sourced from MSCI ESG Research LLC, or its affiliates or information providers (the “ESG Parties”). The Information may only be used for your internal use, may not be reproduced or redisseminated in any form and may not be used as a basis for or a component of any financial instruments or products or indices. Although they obtain information from sources they consider reliable, none of the ESG Parties warrants or guarantees the originality, accuracy and/or completeness, of any data herein and expressly disclaim all express or implied warranties, including those of merchantability and fitness for a particular purpose. None of the MSCI information is intended to constitute investment advice or a recommendation to make (or refrain from making) any kind of investment decision and may not be relied on as such, nor should it be taken as an indication or guarantee of any future performance, analysis, forecast or prediction. None of the ESG Parties shall have any liability for any errors or omissions in connection with any data herein, or any liability for any direct, indirect, special, punitive, consequential or any other damages (including lost profits) even if notified of the possibility of such damages.
Part of this publication may contain Sustainalytics proprietary information that may not be reproduced, used, disseminated, modified nor published in any manner without the express written consent of Sustainalytics. Nothing contained in this publication shall be construed as to make a representation or warranty, express or implied, regarding the advisability to invest in or include companies in investable universes and/or portfolios. The information is provided "as Is" and, therefore Sustainalytics assumes no responsibility for errors or omissions. Sustainalytics cannot be held liable for damage arising from the use of this publication or information contained herein in any manner whatsoever.
© 2021 Vontobel Asset Management, Inc. All Rights Reserved.